Articles, communications, revues… retrouver les dernières publications des membres du laboratoire (classées par année).
PAGE EN COURS DE DÉVELOPPEMENT
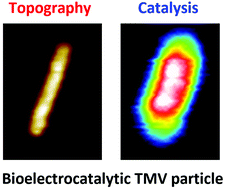
Enzymatic activity of individual bioelectrocatalytic viral nanoparticles: dependence of catalysis on the viral scaffold and its length
Nanoscale Advance article, 2022
The enzymatic activity of tobacco mosaic virus (TMV) nanorod particles decorated with an integrated electro-catalytic system, comprising the quinoprotein glucose-dehydrogenase (PQQ-GDH) enzyme and ferrocenylated PEG chains as redox mediators, is probed at the individual virion scale by atomic force microscopy-scanning electrochemical atomic force microscopy (AFM-SECM). A marked dependence of the catalytic activity on the particle length is observed. This finding can be explained by electron propagation along the viral backbone, resulting from electron exchange between ferrocene moieties, coupled with enzymatic catalysis. Thus, the use of a simple 1D diffusion/reaction model allows the determination of the kinetic parameters of the virus-supported enzyme. Comparative analysis of the catalytic behavior of the Fc-PEG/PQQ-GDH system assembled on two differing viral scaffolds, TMV (this work) and bacteriophage-fd (previous work), reveals two distinct kinetic effects of scaffolding: An enhancement of catalysis that does not depend on the virus type and a modulation of substrate inhibition that depends on the virus type. AFM-SECM detection of the enzymatic activity of a few tens of PQQ-GDH molecules, decorating a 40 nm-long viral domain, is also demonstrated, a record in terms of the lowest number of enzyme molecules interrogated by an electrochemical imaging technique.
Sur le site de l’éditeur : https://doi.org/10.1039/D1NR07445H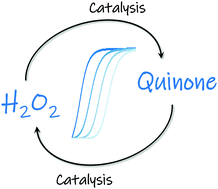
An autocatalytic organic reaction network based on cross-catalysis
Chem. Commun. 57, 11374-11377, 2021
Here we report a simple autocatalytic organic reaction network based on the redox chemistry of quinones and reactive oxygen species. Autocatalysis arises from the cross-activation between the H2O2-catalyzed deprotection of a pro-benzoquinone arylboronic ester probe and the benzoquinone-catalyzed H2O2 production through redox cyling with ascorbate in an aerated buffered solution.
Sur le site de l’éditeur : https://doi.org/10.1039/D1CC05121K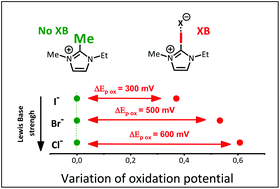
Halogen bonding effect on electrochemical anion oxidation in ionic liquids
Org. Biomol. Chem. 19, 7587-7593, 2021
Three Ionic liquids (ILs) based on an imidazolium core have been compared and used as solvents for the oxidation of various anions. Electrochemical experiments as well as NMR titrations and X-ray diffraction analyses unambiguously confirm the crucial role of non-covalent halogen bonding on the oxidation potentials and consequently the electrochemical window of the respective ILs.
Sur le site de l’éditeur : https://doi.org/10.1039/D1OB01031J
Molecular Electrocatalytic Hydrogenation of Carbonyls and Dehydrogenation of Alcohols
ChemElectroChem 8, 4019-4027, 2021
The development of safe and energy efficient redox processes is key for a future sustainable organic chemistry and energy storage/vector applications. Molecular electrocatalysts have demonstrated their potential in the realm of CO2 reduction, however, successful implementations for the reduction of other carbonyl groups remain sporadic. Building on the reversibility of hydrogenation and dehydrogenation of carbonyls and alcohols, an overview of current molecular electrocatalytic systems is presented. Key mechanistic concepts are emphasized to facilitate the link with more mature schemes in transfer hydrogenation, proton- and CO2-reduction. Thus, this work contributes to future catalyst generation development bridging fundamental aspects of electrochemical bond activation with molecular catalytic concepts in the context of societal challenges of today.
Sur le site de l’éditeur : https://doi.org/10.1002/celc.202100617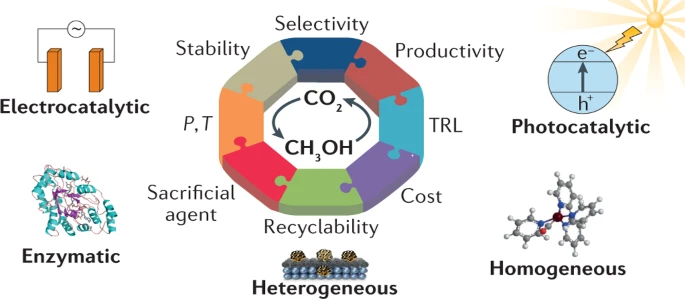
Highlights and challenges in the selective reduction of carbon dioxide to methanol
Nat. Rev. Chem. 5, 564-579, 2021
Carbon dioxide (CO2) is the iconic greenhouse gas and the major factor driving present global climate change, incentivizing its capture and recycling into valuable products and fuels. The 6H+/6e− reduction of CO2 affords CH3OH, a key compound that is a fuel and a platform molecule. In this Review, we compare different routes for CO2 reduction to CH3OH, namely, heterogeneous and homogeneous catalytic hydrogenation, as well as enzymatic catalysis, photocatalysis and electrocatalysis. We describe the leading catalysts and the conditions under which they operate, and then consider their advantages and drawbacks in terms of selectivity, productivity, stability, operating conditions, cost and technical readiness. At present, heterogeneous hydrogenation catalysis and electrocatalysis have the greatest promise for large-scale CO2 reduction to CH3OH. The availability and price of sustainable electricity appear to be essential prerequisites for efficient CH3OH synthesis.
Sur le site de l’éditeur : https://doi.org/10.1038/s41570-021-00289-y
Electrocatalytic and Photocatalytic Reduction of Carbon Dioxide by Earth-Abundant Bimetallic Molecular Catalysts
ChemPhysChem 22, 1835-1843, 2021
Converting CO2 into useful resources by electrocatalysis and photocatalysis is a promising strategy for recycling of the gas and electrification of industries. Numerous studies have shown that multinuclear metal catalysts have higher selectivity and catalytic activity than monometallic catalysts due to the synergistic effects between the metal sites. In this review, we summarize some of the recent progress on the electrocatalytic and photocatalytic reduction of CO2 by earth-abundant bimetallic molecular catalysts.
Sur le site de l’éditeur : https://doi.org/10.1002/cphc.202100330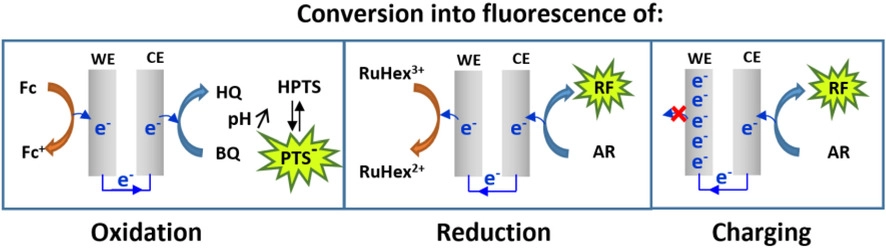
Real-time Conversion of Electrochemical Currents into Fluorescence Signals Using 8-Hydroxypyrene-1,3,6-trisulfonic Acid (HPTS) and Amplex Red as Fluorogenic Reporters
ChemElectroChem 8, 2298-2307, 2021
This paper elaborates on an instrumental scheme for translating electrochemical reactions occurring at an electrode under potentiostatic control into fluorescent signals. We examine the performance enhancement of this device using recently introduced water-soluble fluorescent reporter systems, either the pH-sensitive 8-hydroxypyrene-1,3,6-trisulfonic acid (HPTS) system or the electrofluorogenic Amplex Red species. The conversion of stationary and transient, cathodic and anodic electrochemical currents into fluorescence is demonstrated. We show that pure capacitive currents can also be converted to fluorescence by our setup, highlighting the rarely observed coupling between a faradaic reaction at one electrode and capacitive charging at another.
Sur le site de l’éditeur : https://doi.org/10.1002/celc.202100517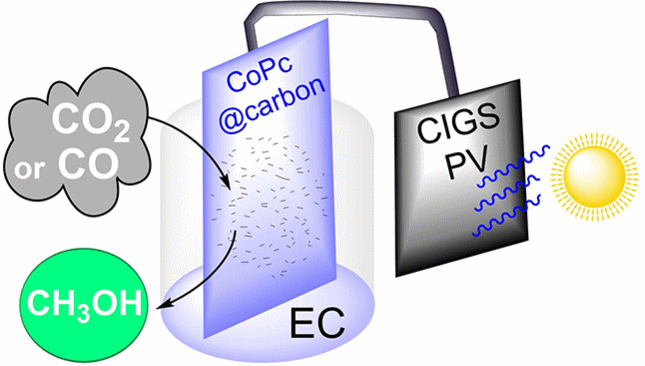
Carbon Dioxide Reduction to Methanol with a Molecular Cobalt-Catalyst-Loaded Porous Carbon Electrode Assisted by a CIGS Photovoltaic Cell
ChemPhotoChem 5, 705-710, 2021
Conversion of CO2 into valuable compounds, including fuels, with renewable energy sources and sustainable compounds is a challenge addressed by artificial photosynthesis research. In particular, the application of solar assisted electrochemical (EC) processes, in which electrons are furnished by a photovoltaic (PV) cell, is a promising approach. A PV-EC system is described, consisting of a CIGS (copper indium gallium selenide) PV unit linked to a carbon electrode loaded with cobalt phthalocyanine as molecular catalyst, able to achieve the CO2 reduction to CO and then to methanol in aqueous media with limited bias voltage. Using CO as starting material, a partial current density of ca. 0.6 mA cm−2 for methanol is obtained at a bias voltage corresponding to a low 240 mV overpotential. Remarkably, the liquid fuel production can be sustained for at least 7 h. Under ideal conditions, the CO2-to-CH3OH reaction shows a global Faradaic efficiency of 28 %.
Sur le site de l’éditeur : https://doi.org/10.1002/cptc.202100035
A Pioneering Career in Electrochemistry: Jean-Michel Savéant
ACS Catal. 11, 3224-3238, 2021
Prof. Jean-Michel Savéant sadly passed on August 16, 2020. We would like to honor his memory, his tremendous contribution to electrochemistry, and its use for a general understanding of the laws of physical chemistry. In this review, we highlight his decisive role in the foundation of molecular electrochemistry. We also present his major achievements in the field of molecular and biomolecular catalysis. Finally, we review his unique contribution to dissociative electron transfers and to the electrochemical approach of proton-coupled electron transfers. This shows how various concepts rigorously established and experimentally validated assemble to each other to enlighten complex systems.
Sur le site de l’éditeur : https://doi.org/10.1021/acscatal.0c05632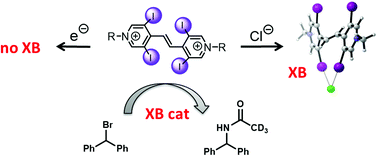
Towards redox-switchable organocatalysts based on bidentate halogen bond donors
Phys. Chem. Chem. Phys. 23, 4344-4352, 2021
Redox-active bidentate halogen bond donors based on halopyridinium groups as halogen-bond donating units were synthesized and their structures were elucidated by X-ray diffraction analyses and DFT calculations. Via reversible twofold reduction, these dicationic species can be transformed to neutral compounds which should be much weaker Lewis acids. The corresponding electrochemical data were obtained, and CV as well as UV-vis and NMR techniques were also used to determine binding constants of these halogen bond donors to halides. While all titrations agree on the relative order of binding strengths (with chloride being bound strongest), there are marked deviations in the overall affinity constants which are discussed. In contrast to earlier azo-bridge analogues, the ethylene-linked variants presented herein do not oxidize halides, and thus the novel halogen bond donors could also be used as Lewis acidic organocatalysts in a halide abstraction benchmark reaction, yielding a performance similar to bis(haloimidazolium)-derived catalysts.
Sur le site de l’éditeur : https://doi.org/10.1039/D0CP06612E